When searching for information about Nutrafol supplement side effects, many consumers find themselves in a frustrating position. You’ve likely experienced it yourself—clicking through multiple websites only to discover vague claims, incomplete information, or content that seems suspiciously promotional. This information gap isn’t accidental; it stems from a complex landscape where verified medical data about supplement side effects is often difficult to access reliably.
The challenge with researching supplement side effects like those potentially associated with Nutrafol is that supplements operate in a regulatory gray area. Unlike pharmaceutical drugs that undergo rigorous FDA testing before market approval, dietary supplements like Nutrafol follow different regulatory pathways. This means comprehensive side effect data isn’t always systematically collected or publicly available, leaving consumers to navigate a maze of anecdotal reports and incomplete information.
In this article, we’ll help you understand why verified information about Nutrafol supplement side effects is scarce, how to identify reliable sources when they do exist, and most importantly—what steps you can take to protect your health while using any dietary supplement. You’ll learn how to recognize legitimate side effect reporting versus marketing spin, understand the limitations of current supplement regulation, and discover practical strategies for monitoring your own response to supplements.
How Supplement Regulation Creates Information Gaps
Why Nutrafol Side Effect Data Is Limited Compared to Prescription Medications
The fundamental reason for scarce Nutrafol supplement side effects information lies in how supplements are regulated. While pharmaceutical drugs must prove safety and efficacy through clinical trials before reaching consumers, supplements like Nutrafol follow a different pathway under the Dietary Supplement Health and Education Act (DSHEA) of 1994. This law places the burden of proof on the FDA to demonstrate a supplement is unsafe after it’s already on the market, rather than requiring manufacturers to prove safety beforehand.
Without mandatory pre-market safety trials, comprehensive side effect databases don’t exist for supplements as they do for prescription medications. Nutrafol’s manufacturer may collect some adverse event reports, but they aren’t required to publish comprehensive side effect profiles. This creates significant information gaps for consumers trying to make informed decisions about potential Nutrafol supplement side effects.
How to Identify When Supplement Information Is Unreliable
Many websites discussing Nutrafol supplement side effects contain red flags that indicate unreliable information. Be wary of sites that:
– Make absolute claims like “no side effects ever reported”
– Lack citations to scientific studies or regulatory documents
– Use medical terminology incorrectly
– Exist primarily to sell the product being discussed
– Present anecdotal experiences as scientific evidence
Reliable health information should transparently acknowledge uncertainties and limitations in available data. When researching potential Nutrafol supplement side effects, prioritize sources that clearly state what is known versus what remains uncertain in the medical community.
Recognizing Potential Supplement Reactions
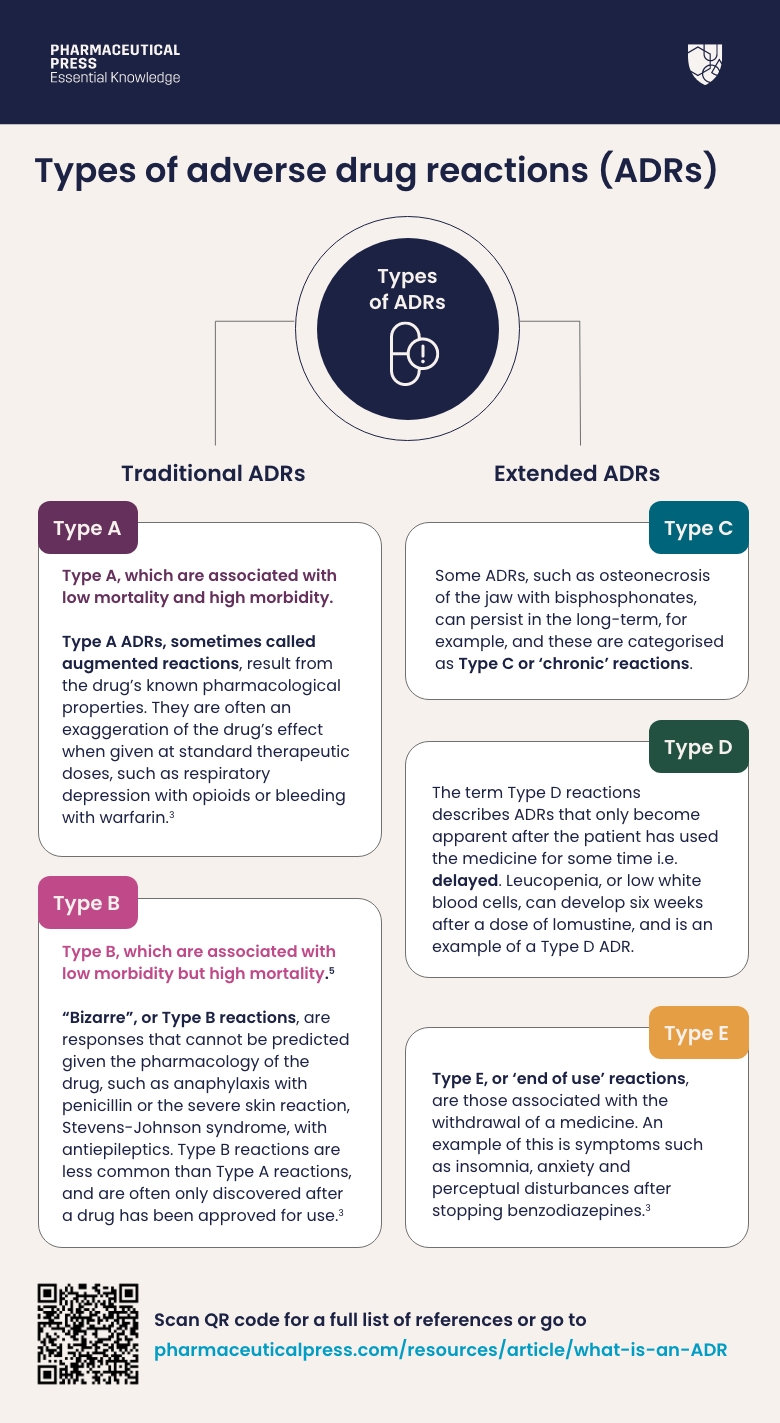
Differentiating Between Expected Responses and True Side Effects
When using any supplement including Nutrafol, it’s crucial to distinguish between normal physiological responses and actual adverse reactions. Some users might experience temporary adjustments like:
– Mild digestive changes during initial use
– Subtle shifts in energy levels as the body adapts
– Temporary changes in sleep patterns
These aren’t necessarily side effects but rather your body adjusting to new compounds. True side effects typically involve:
– Symptoms that worsen over time rather than improving
– Reactions that significantly impact daily functioning
– Physical manifestations outside normal physiological ranges
Monitoring your body’s response systematically—perhaps through a symptom journal—can help differentiate between normal adjustment periods and concerning side effects that warrant discontinuation or medical consultation.
How to Document and Report Supplement Reactions
If you suspect you’re experiencing side effects from Nutrafol or any supplement, proper documentation is essential. Record:
– The exact date and time symptoms began
– Specific description of symptoms (not just “felt bad”)
– Dosage and timing of supplement intake relative to symptoms
– Any other medications, supplements, or significant dietary changes
This information becomes valuable if you need to consult a healthcare provider or report the reaction to appropriate authorities. The FDA’s MedWatch program accepts adverse event reports for dietary supplements, though consumer reporting rates remain low compared to prescription medications.
Safety Strategies for Supplement Users
Consultation Protocols Before Starting Any New Supplement
Before incorporating Nutrafol or similar supplements into your routine, implement these safety measures:
– Schedule a dedicated appointment with your physician specifically to discuss supplements (not just mention it at the end of a regular checkup)
– Bring the exact product packaging or full ingredient list to your appointment
– Disclose all other supplements and medications you’re taking
– Ask specifically about potential interactions with your existing health conditions
Many potential side effects occur due to interactions between supplements and medications or underlying health conditions that might not be obvious to the consumer. A healthcare provider can help identify these risk factors before they become problems.
Creating Your Personal Supplement Safety Plan
Develop a structured approach to monitoring your response to supplements:
- Start low: Begin with the lowest possible dose to assess initial tolerance
- Introduce one at a time: Avoid starting multiple new supplements simultaneously
- Track systematically: Use a dedicated journal or app to record daily observations
- Set review points: Schedule specific dates to objectively evaluate benefits versus any negative effects
- Establish exit criteria: Determine in advance what symptoms would prompt you to discontinue use
This methodical approach helps isolate whether any emerging symptoms are genuinely related to the supplement rather than other factors in your environment or health status.
Navigating Supplement Information Responsibly
Evaluating Research Claims About Nutrafol Ingredients

When examining claims about Nutrafol supplement side effects (or lack thereof), scrutinize references to scientific studies:
– Check if cited research actually involved the complete Nutrafol formula or just individual ingredients
– Determine whether studies were conducted on humans or only in laboratory settings
– Look for independent replication of findings (studies funded by the manufacturer warrant extra scrutiny)
– Verify whether research examined long-term use effects, not just short-term trials
Many supplement websites conflate research on individual components with evidence about the complete product’s safety profile—a significant distinction when assessing potential side effects.
Understanding the Limitations of User Reviews
While online reviews about Nutrafol supplement side effects might seem helpful, they come with significant limitations:
– Selection bias: People who experience strong reactions (positive or negative) are more likely to post reviews
– No medical verification: Anyone can claim any symptom without professional confirmation
– Dosage variations: Reviewers may not be using the product as directed
– Timeframe issues: Long-term effects rarely appear in early reviews
Treat user reviews as potential signals to investigate further rather than definitive evidence of side effects. If multiple reviewers mention similar concerns, that might indicate an area worth discussing with your healthcare provider.
When to Seek Professional Guidance
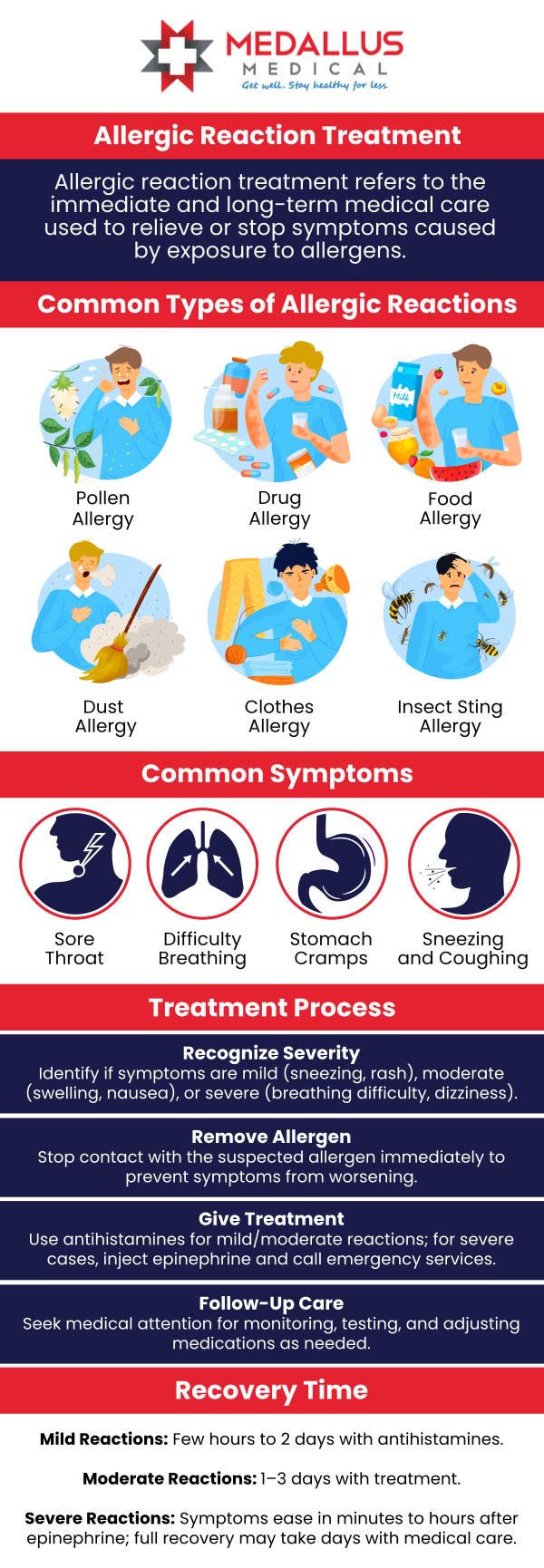
Recognizing Red Flag Symptoms That Require Immediate Attention
Certain symptoms potentially associated with supplements like Nutrafol should prompt immediate medical consultation:
– Severe or persistent gastrointestinal distress
– Unexplained skin reactions or rashes
– Significant changes in heart rate or blood pressure
– Severe headaches or neurological symptoms
– Signs of allergic reaction (swelling, difficulty breathing)
Don’t dismiss these as “just part of the adjustment period.” Your body might be signaling a serious reaction that requires professional evaluation. Remember that supplement ingredients can interact with underlying health conditions you might not even be aware of.
Building an Ongoing Dialogue With Your Healthcare Team
Supplement safety isn’t a one-time conversation. Establish regular check-ins with your healthcare provider to discuss:
– Any changes in how you feel since starting the supplement
– Whether you’ve noticed benefits matching the product’s claims
– How the supplement fits within your overall health strategy
– Whether continued use remains appropriate based on your evolving health status
This ongoing dialogue transforms supplement use from a potentially risky experiment into a monitored component of your healthcare strategy.
Final Note: While comprehensive data on specific Nutrafol supplement side effects remains limited due to regulatory gaps in the supplement industry, you can take concrete steps to protect your health. By approaching supplements with the same caution you’d use with medications—researching thoroughly, consulting professionals, monitoring your response, and reporting concerns—you transform from a passive consumer into an informed participant in your healthcare journey. Always remember that when it comes to your health, incomplete information shouldn’t be mistaken for safety assurance. When in doubt about potential side effects, consult a healthcare professional who can evaluate your individual health context rather than relying solely on internet information.


